the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Evaluation of human risks of surface water and groundwater contaminated with Cd and Pb in the southern El-Minya Governorate, Egypt
Salman A. Salman
Ahmed A. Asmoay
Amr El-Gohary
Hassan Sabet
Water pollution with cadmium (Cd) and lead (Pb) is a worldwide concern because of their health impact. Determination of their concentrations and potential human health risks in surface water and groundwater in the southern El-Minya Governorate, Egypt, is the main aim of this study. Fifty-five samples were collected, 30 surface water samples and 25 groundwater samples. The samples were analyzed using atomic absorption spectrometry to determine Cd and Pb contents. Their levels in surface water and groundwater exceeded the maximum allowable level for drinking water set by the World Health Organization (WHO). The hazard quotient showed that the surface water and groundwater may pose a health risk to residents, especially to children.
Water resource pollution is becoming a worldwide problem. To protect the environment and public health, it is important to have precise knowledge of the concentration and type of water pollutants, especially heavy metals. Cadmium (Cd) and lead (Pb) are among the most important chemical pollutants that threaten water quality for different uses. Monitoring their concentrations in water is critical for protecting ecological and human health because of their harmful effects and persistence (Nazar et al., 2012).
Cd is a major environmental concern and is ranked as the sixth most important toxic substance (ATSDR, 1997). It is released into the aqueous system from metal plating, smelting, mining, cadmium–nickel batteries, phosphate fertilizers, paint industries, pigments, and alloy industries, as well as from sewage (Kadirvalu and Namasivayam, 2003). The nervous system appears to be the most sensitive target of Cd toxicity. Cd exposure can produce a wide variety of acute and chronic effects in humans, such as renal failure, lung insufficiency, bone lesions, and hypertension (Sun and Li, 2011).
Pb is the most common environmental contaminant (Chiang et al., 2012). It does not undergo degradation or decomposition. Thus, its long persistence in the environment exacerbates its threat to human health. Pb is used in many industries, including lead smelting and processing, battery manufacturing, pigments, solder, plastics, cable sheathing, fuels, ammunition, and ceramics. Due to urbanization, Pb and other metals are regularly discharged into fields, water, and soils through sewage sludge, urban runoff, and automobile exhaust (Elnazer et al., 2015). Pb absorbed by the human body disturbs many processes and is harmful to organs such as the heart, bones, and nervous system (Bruce et al., 2012).
The application of a human health risk assessment model has many advantages in comparison with drinking water guidelines such as the WHO guidelines, because it takes into consideration the age, body weight, metal concentration, exposure duration, and daily intake (USEPA, 2011). So, quantifying human risk (carcinogenic and non-carcinogenic risks) of Cd and Pb to children and adults is important. The hazard quotient (HQ) is extensively used to characterize the carcinogenic and non-carcinogenic health effects of toxic metals by comparison of their exposure effects to a reference dose (RfD) (Qu et al., 2012). This was documented in several studies by taking into consideration exposure scenarios of metal intake through contaminated water (Muhammad et al., 2011; Shah et al., 2012; Dou and Li, 2012). So, the objective of this study is the assessment of the potential human health risks for Cd and Pd in the surface water and groundwater systems in the western part of the River Nile between Abu Qurqas and Dyer Mawas districts, southern El-Minya Governorate, Egypt.
2.1 Location
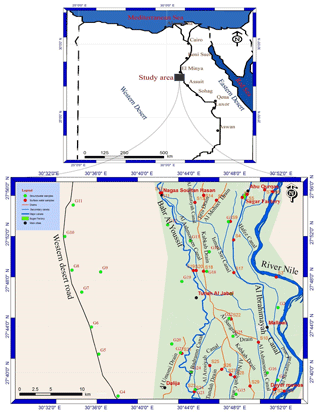
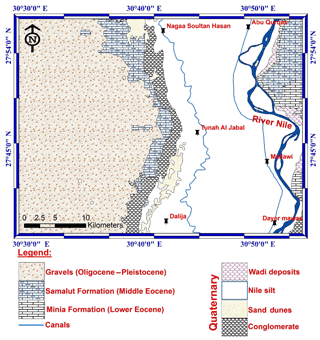
The study area occupied the middle part of the Nile Valley between longitudes 30∘29′ and 30∘54′ E and latitudes 27∘37′ and 27∘56′ N (Fig. 1). It is bounded by the River Nile from the east and the calcareous plateau in the west between Abu Qurqas northward and Dyer Mawas in the south. The water resources in the study area are represented by the River Nile, canals, drains, as well as groundwater (Fig. 1). The stratigraphic succession (Fig. 2) in the El-Minya area is represented by Tertiary and Quaternary sedimentary rocks (Said, 1981). The main aquifer in the study area is represented by Pleistocene sediments which are composed of sand and gravel of different sizes with some clay intercalation (Sadek, 2001). The aquifer is semi-confined in the old cultivated land (in the eastern part of the study area) and unconfined in the desert fringes (in the western part of the study area). The groundwater generally flows from the southern part to the northern part of the study area. The aquifer is recharged by Nile water, irrigation systems, drains, agricultural infiltration, and vertical upward from the deeper saline aquifers (Abdalla et al., 2009). The main sources of water for different purposes in the study area are the Nile, canals, drains, and groundwater. The study area contains many industrial zones, agricultural activities, and urban areas.
2.2 Sampling and analyses
In November 2014, 30 water samples were collected from surface water resources in the study area (Fig. 1 and Table 1). In addition, 25 groundwater samples were collected from the Quaternary aquifer (Fig. 1). Pre-rinsed polypropylene bottles were filled with the samples, acidified (pH < 2) with nitric acid to prevent precipitation and microbial activity and sorption losses to container walls, and were sealed tightly. At the lab the samples were filtered through filter paper (Whatman no. 42) and digested with nitric acid (APHA, 1995). Samples were analyzed using an atomic absorption spectrometer instrument (model: Perkin Elmer 400) in the National Research Centre Laboratories.
For health risk assessment, non-carcinogenic (HQnc) and carcinogenic (HQc) hazard quotients for each contaminant were calculated according to the following equations (Kelepertzis, 2014):
where CDI, C, IR, ED, EF, BW, AT, and RfD represent chronic daily intake (mg kg−1 d−1), concentration of metal in water (mg L−1), average daily intake rate (2 L d−1 for adults and 1.2 L d−1 for children), exposure duration (15 years), exposure frequency (350 d), body weight (70 kg for adults and 28 kg for children), average time (ED*365 d for non-carcinogenic and lifetime*365 for carcinogenic risk) and toxicity reference dose. According to the USEPA (2011), the RfD for Cd and Pb are 0.0005 and 0.055 mg kg−1 d−1, respectively, and the slope factor (SF) is 0.38 and 0.055 mg kg−1 d−1, respectively. The average lifetime for an adult is 65, and 6.5 years for children.
3.1 Surface water
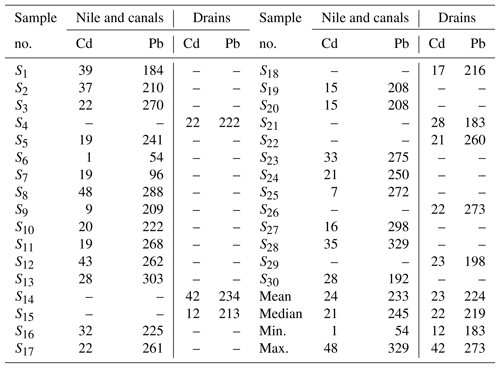
The River Nile and its tributaries (canals and drains) are the main source of water in Egypt, especially for the governorates located on the river banks and on its branches. Cd concentrations (Table 2), ranging from 1 to 48 µg L−1, exceeded the permissible limit (3 µg L−1) for drinking water according to the WHO (2011). Excess Cd could accumulate in the kidneys and remains for many years, causing irreversible kidney damage (Goyer, 1996). The number of kidney patients in the study area is presumed to have increased from 10 patients per million in 1974 to about 165 patients per million in 1995, and in 2005 it was 260 patients per million in El-Minya Governorate (El-Minshawy and Kamel, 2006). The highest concentration of Cd was recorded in sample number S8, 48 µg L−1, close to Abu Qurqas Sugar factory (Al-Shiekh Sharf canal). The lowest concentration was recorded in sample number S6 (1 µg L−1), which was collected from the River Nile. These results are in line with those obtained by Toufeek (2011), who recorded an average Cd concentration of 12.5 µg L−1 at Aswan (southern Egypt), while Melegy et al. (2014) found that the Cd levels in the collected samples from Sohag Governorate were around 16 µg L−1. Osman and Kloas (2010) mentioned that the average Cd concentration in the River Nile at Assuit was 6 µg L−1.
Pb concentration ranged from 54 to 329 µg L−1 in the studied surface water samples (Table 2). The concentration of Pb passed the permissible limit (10 µg L−1) for drinking water according to the WHO (2011). Pb adsorbed by the human body disturbs many body processes and is harmful to many organs and tissues such as the heart, bones, and nervous system (Bruce et al., 2012). The highest concentration of Pb was recorded in sample number S28 (329 µg L−1) from Al-Nasriyah canal, while sample number S6, which was collected from the River Nile in Abu Qurqas district, contained the lowest concentration (54 µg L−1). These results are in agreement with those obtained by Toufeek (2011), who reported about 214 µg L−1 Pb in the River Nile at Aswan. In addition, Osman and Kloas (2010) proved that the average Pb concentration in the River Nile at Assuit was nearly 24 µg L−1.
Agricultural activities are considered the most important source of Cd and Pb, where the used super-phosphate fertilizers in the study area contain about 8.5 and 16 ppm Cd and Pb, respectively (Asmoay, 2017). In addition, vehicle exhaust, industrial activities, and urban runoff contribute to a great extent to the pollution of water with these metals (Elnazer et al., 2018).
3.2 Groundwater
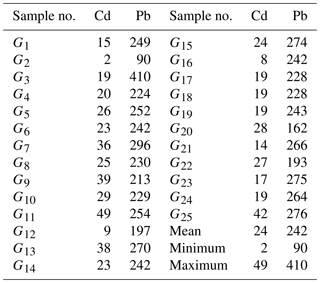
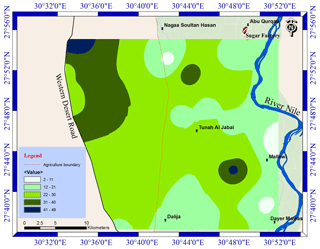
Groundwater is the second important water resource in the study area, and it is used for irrigation as well as for domestic use and drinking in desert fringes and some villages which do not have access to drinking water. The groundwater samples exhibit a relatively wide range of the Cd level, varying from 2 to 49 µg L−1 with a mean value of 24 µg L−1 (Table 3). The groundwater Cd level decreased eastward (Fig. 3), probably due to mixing with the surface water from the River Nile and the role of the silty clay layer in the adsorption of Cd, which prevent it from reaching the aquifers (Asmoay, 2017). However, there were three hotspots of Cd identified, resulting from intensive human activities and fuel stations (Asmoay, 2017). The Cd hotspot in the northwestern part of the study area adjacent to the western desert road is vulnerable as a result of the unconfined condition of the aquifers (Asmoay, 2017).
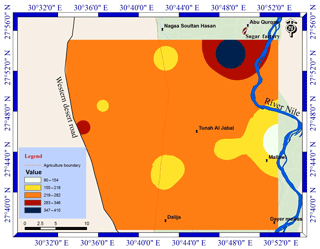
Also, the measured Pb contents of the analyzed groundwater samples show a relatively wide range varying from 90 to 410 µg L−1 with an average of 242 µg L−1 (Table 3). The marked high level of Pb content implies that the anthropogenic activities (agricultural and urban runoff) are the main sources of Pb. The results are in agreement with Melegy et al. (2014), who mentioned that Cd and Pb concentrations in the groundwater of Sohag were around 21 and 383 µg L−1, respectively. The Pb level in the groundwater of the study area was increased in Abu Qurqas district, resulting from the effect of the sugar factory, cesspits, and fuel stations (Asmoay, 2017) as shown in Fig. 4.
All the samples were unsuitable for drinking purposes where they possess Cd and Pb values above the permissible limit of 3 µg L−1 for Cd and 10 µg L−1 for Pb (WHO, 2011). On the other hand, surface water and groundwater were suitable for irrigation purposes according to NAS-NAE (1973), since they contain less than 10 and 5000 mg L−1 of Cd and Pb, respectively.
3.3 Health risk assessment
Water from the River Nile and canals as well as groundwater are used for drinking and domestic purposes. The applied treatment techniques (flocculation and coagulation with alum) in drinking water stations (Donia, 2007) in the study area are effective for the removal of toxic metals bound to suspended solids (Fatoki and Ogunfowokan, 2002). However, it was observed that a great number of residents in rural areas use groundwater from hand pumps for drinking because they do not have access to the tap water. El-Minshawy and Kamel (2006) mentioned that the use of unsafe water for drinking contributes up to 71.8 % of the renal failure in the study area. Therefore, health risk assessments of surface water and groundwater were also carried out in this study. The results of HQnc and HQc due to metal exposure in surface water and groundwater samples are provided in Tables 4 and 5.
Table 4Statistical parameters of non-carcinogenic and carcinogenic health risks for surface water samples.
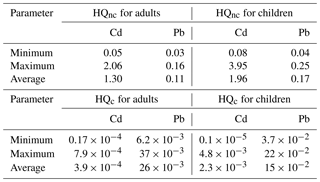
Table 5Statistical parameters of non-carcinogenic and carcinogenic health risks for groundwater samples.
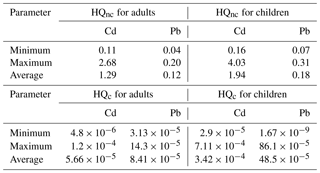
The calculated HQnc average values for Cd in surface water for adults and children were 1.30 and 1.96, respectively, while HQnc average values for Pb were 0.11 and 0.17 (Table 4). On the other hand, the calculated HQnc average values for Cd in groundwater for adults and children were 1.29 and 1.94, respectively, while HQnc average values for Pb were 0.12 and 0.18 (Table 5). According to the USEPA (2011), Cd can cause non-carcinogenic health problems because its HQnc values were more than 1, while Pb presence in water had no adverse health impacts (HQnc < 1), although its concentration did exceed WHO (2011) guidelines.
The calculated HQc values for Cd in the surface water for adults ranged from to and for children from to (Table 4). The calculated HQc values for Pb varied from to for adults and varied from to for children (Table 4), while the calculated HQc values for Cd in the groundwater ranged from to for adults and from to for children (Table 5). The calculated HQc values for Pb varied between and for adults and varied from to for children (Table 5). According to USEPA (2011) recommended values (HQc < 10−6), the studied surface water and groundwater consumption could cause carcinogenic health risks in adults and children.
Cd and Pb contents of the studied samples from the River Nile, canals, drains as well as groundwater exceeded the permissible limits for drinking water and could have adverse, carcinogenic, impacts.
No data sets were used in this article.
All the authors contributed equally in all the article steps. All the authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.
The authors gratefully acknowledge the National Research Centre for funding this research as a Ph.D. internal project, and the grant no. is (8/5/9) to support Ahmed A. Asmoay to do the lab work.
This research has been supported by the National Research Centre (grant no. 8/5/9).
This paper was edited by Luuk Rietveld and reviewed by two anonymous referees.
Abdalla, F. A., Ahmed, A. A., and Omer, A. A.: Degradation of groundwater quality of quaternary aquifer at Qena, Egypt, J. Environ. Studies, 1, 19–32, 2009.
APHA: Standard methods for the examination of water and wastewater, 19th edn., American Public Health Association, Washington, D. C., 1467 pp., 1995.
Asmoay, A. S. A.: Hydrogeochemical Studies on the Water Resources and Soil Characteristics in the Western Bank of the River Nile between Abu Qurqas and Dayr Mawas, El Minya Governorate, Egypt, Ph.D. thesis, Fac. Sci., Al-Azhar University, Egypt, 2017.
ATSDR: Toxicological profile for Cadmium, Agency for Toxic Substances and Disease Registry, Georgia, 430 pp., 1997.
Bruce, S. G., Zarema, A., and Igor, M. G.: Analysis of Lead toxicity in human cells, BMC Genomics, 13, 344, https://doi.org/10.1186/1471-2164-13-344, 2012.
Chiang, Y. W., Santos, R. M., Ghyselbrecht, K., Cappuyns, V., Martens, J. A., Swennen, R., Gerven, T. V., and Meesschaert, B.: Strategic selection of an optimal sorbent mixture for in-situ remediation of heavy metal contaminated sediments: Framework and case study, J. Environ. Manage., 105, 1–11, 2012.
Donia, N.: Survey of Potable Water Quality Problems in Egypt, Eleventh International Water Tech. Conf., IWTC11 Sharm El-Sheikh, Egypt, 1049–1058, 2007.
Dou, M. and Li, C.: Health risk assessment of Cadmium pollution emergency, Energy Procedia, 16, 290–295, 2012.
El-Minshawy, O. and Kamel, E. G.: Renal replacement therapy and increased risk of cardiovascular, Egypt. J. Hosp. Med., 22, 29–38, 2006.
Elnazer, A. A., Salman, S. A., Seleem, E. M., and Abu El Ella, E. M.: Assessment of Some Heavy Metals Pollution and Bioavailability in Roadside Soil of Alexandria-Marsa Matruh Highway, Egypt, Int. J. Ecol., 2015, 689420, https://doi.org/10.1155/2015/689420, 2015.
Elnazer, A. A., Mostafa, A., Salman S. A., Seleem, E. M., and Al-Gamal, A. G.: Temporal and spatial evaluation of the River Nile water quality between Qena and Sohag Cities, Egypt, B. Natl. Res. Centre, 42, 3, https://doi.org/10.1186/s42269-018-0005-6, 2018.
Fatoki, O. S. and Ogunfowokan, A. O.: Effect of coagulant treatment on the metal composition of raw water, Water SA, 28, 293–297, 2002.
Goyer, R. A.: Toxic effects of metals: mercury, in: Casarett and Duoll's toxicology, The Basic Science of Poisons, edited by: Klassen C. D., McGraw-Hill, New York, NY, 709–712, 1996.
Kadirvalu, K. and Namasivayam, C.: Activated carbon from coconut coirpith as metal adsorbent: Adsorption of Cd (II) from Aqueous Solution, Adv. Environ. Res., 7, 471, https://doi.org/10.1016/S1093-0191(02)00018-7, 2003.
Kelepertzis, E.: Investigating the sources and potential health risks of environmental contaminants in the soils and drinking waters from the rural clusters in Thiva area (Greece), Ecotoxicol. Environ. Safe., 100, 258–265, 2014.
Melegy, A. A., Shaban, A. M., Hassaan, M. M., and Salman, S. A.: Geochemical Mobilization of Some Heavy Metals in Water Resources and Their Impact on Human Health in Sohag Governorate, Egypt, Arab. J. Geosci., 7, 4541–4552, 2014.
Muhammad, S., Shah, M. T., and Khan, S.: Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan, MicroChem J., 98, 334–343, 2011.
NAS-NAE: Water quality criteria. Report prepared by committee of water quality on request of U.S. Environmental Protection Agency, National Academy of Science and National Academy of Engineering, Washington, D. C., USA, 594 pp., 1973.
Nazar, R., Iqbal, N., Masood, A., Iqbal, M., Khan, R., Syeed, S., and Khan, N. A.: Cadmium toxicity in plants and role of mineral nutrients in Its Alleviation, Am. J. Plant Sci., 3, 1476–1489, 2012.
Osman, A. G. M. and Kloas, W.: Water quality and heavy metal monitoring in water, sediments, and tissues of the African Catfish Clariasgariepinus (Burchell, 1822) from the River Nile, Egypt, J. Environ. Prot., 1, 389–400, 2010.
Qu, C. S., Ma, Z. W., Yang, J., Liu, Y., Bi, J., and Huang, L.: Human exposure pathways of heavy metals in alead-zinc mining area, Jiangsu Province, China, PLoS One, 7, e46793, https://doi.org/10.1371/journal.pone.0046793, 2012.
Sadek, M.: Istopic criteria for upward leakage in the alluvial aquifer in north El Minia district, Egypt, Egyptian Geology Survey and Mining Authority, 19 pp., 2001.
Said, R.: Geological evaluation of the Nile, Springer-Verlag, NY, 151 pp., 1981.
Shah, T., Ara, J., Muhammad, S., Khan, S., and Tariq, S.: Health risk assessment via surface water and subsurface water consumption in the mafic and ultra-mafic terrain, Mohmand agency, Northern Pakistan, J. Geochem. Explor., 118, 60–67, 2012.
Sun, H. and Li, L.: Investigation of distribution for trace Lead and Cadmium in chinese herbal medicines and their decoctions by graphite furnace Atomic Absorption Spectrometry, Am. J. Anal. Chem., 2, 217–222, 2011.
Toufeek, M. E. F.: Distribution of Cadmium and Lead in Aswan reservoir and River Nile water at Aswan, World Appl. Sci. J., 13, 369–375, 2011.
USEPA: Exposure Factors Handbook, United States Environmental Protection Agency, Washington, D. C., EPA/600/R-09/052F, 2011.
WHO: Guideline for drinking water quality, World Health Organization, 4th edn., 564 pp., 2011.